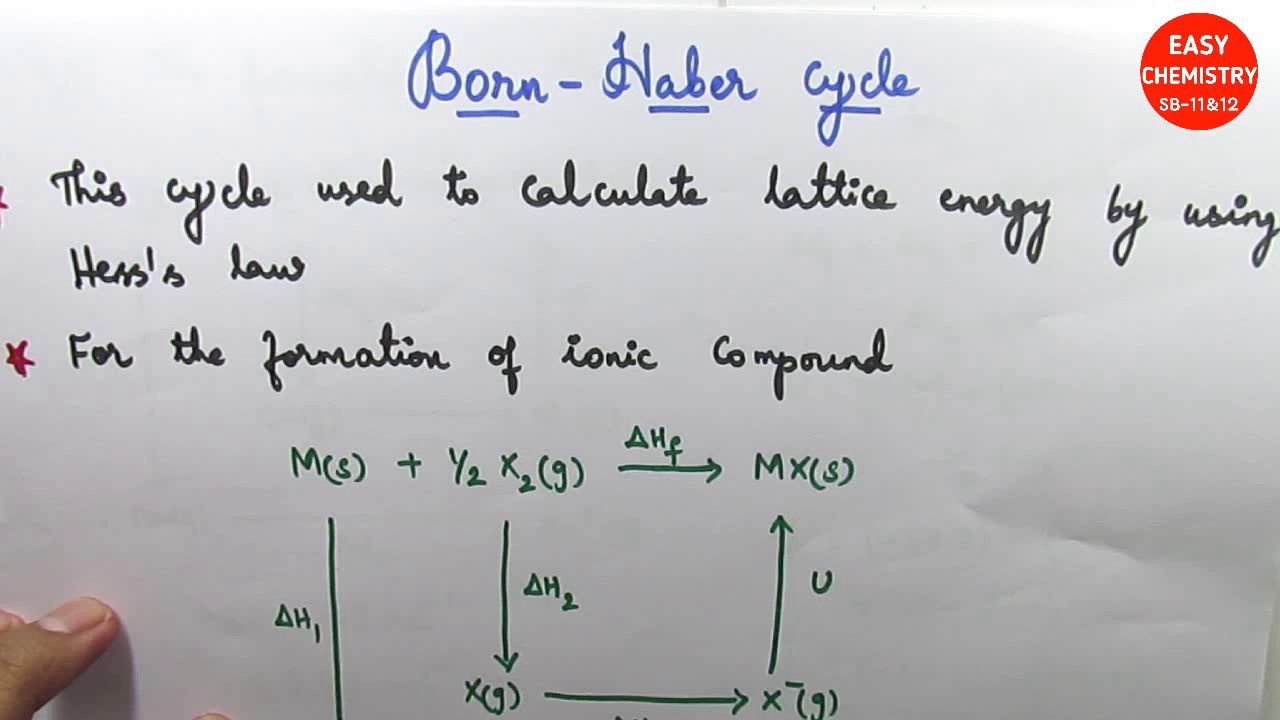
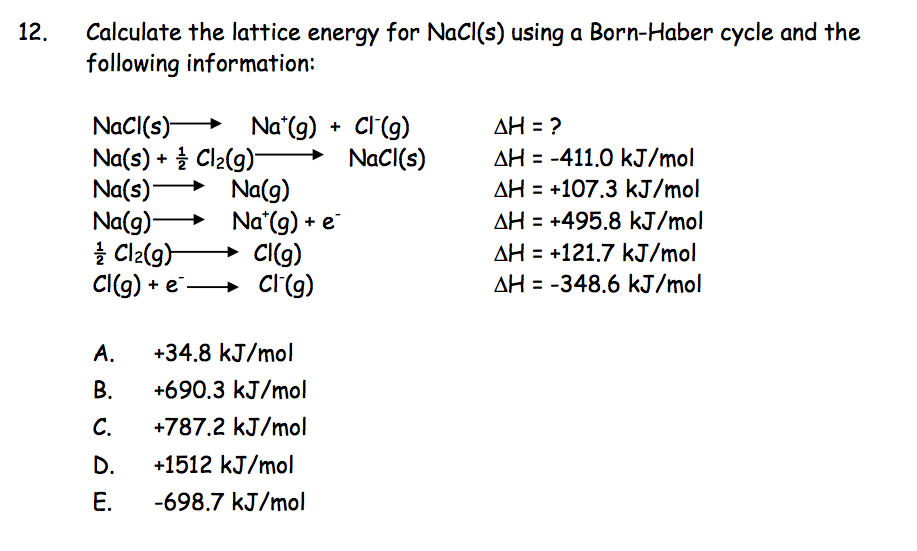
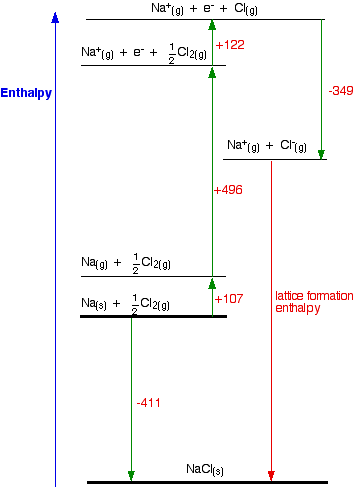
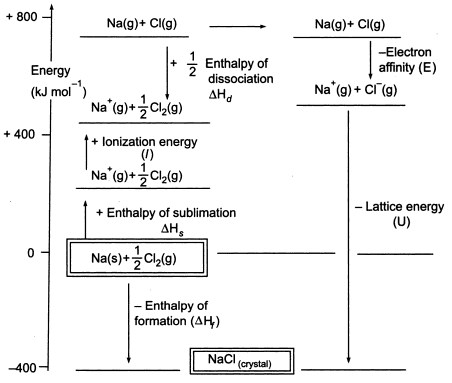
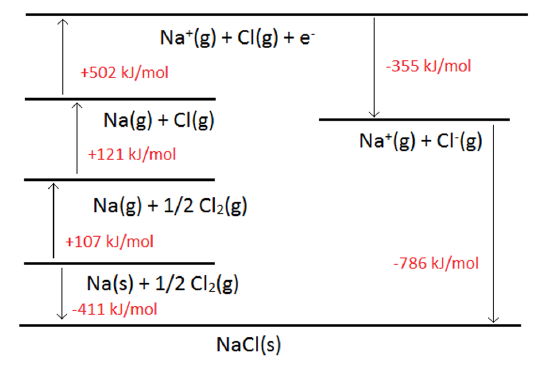
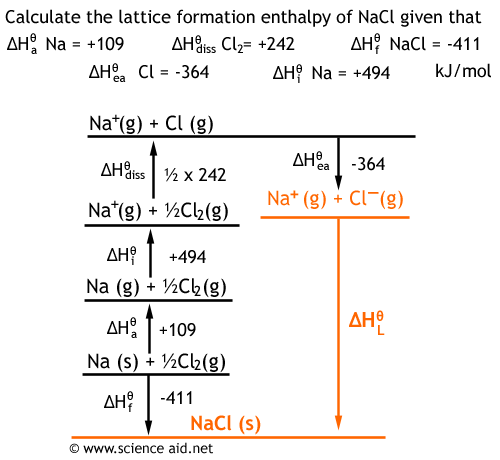
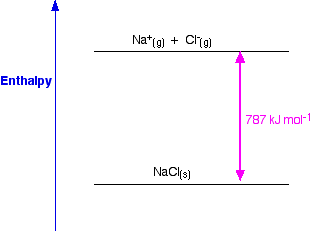
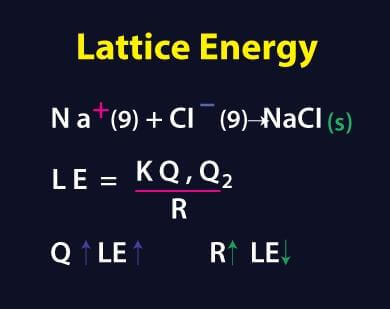
Standard heat of formation of KI is - 78.31 kcal mol^-1 . Calculate its lattice energy from following information: IE (K) = 4.3 eV EA (I) = - 73.4 kcal mol^-1 Bond
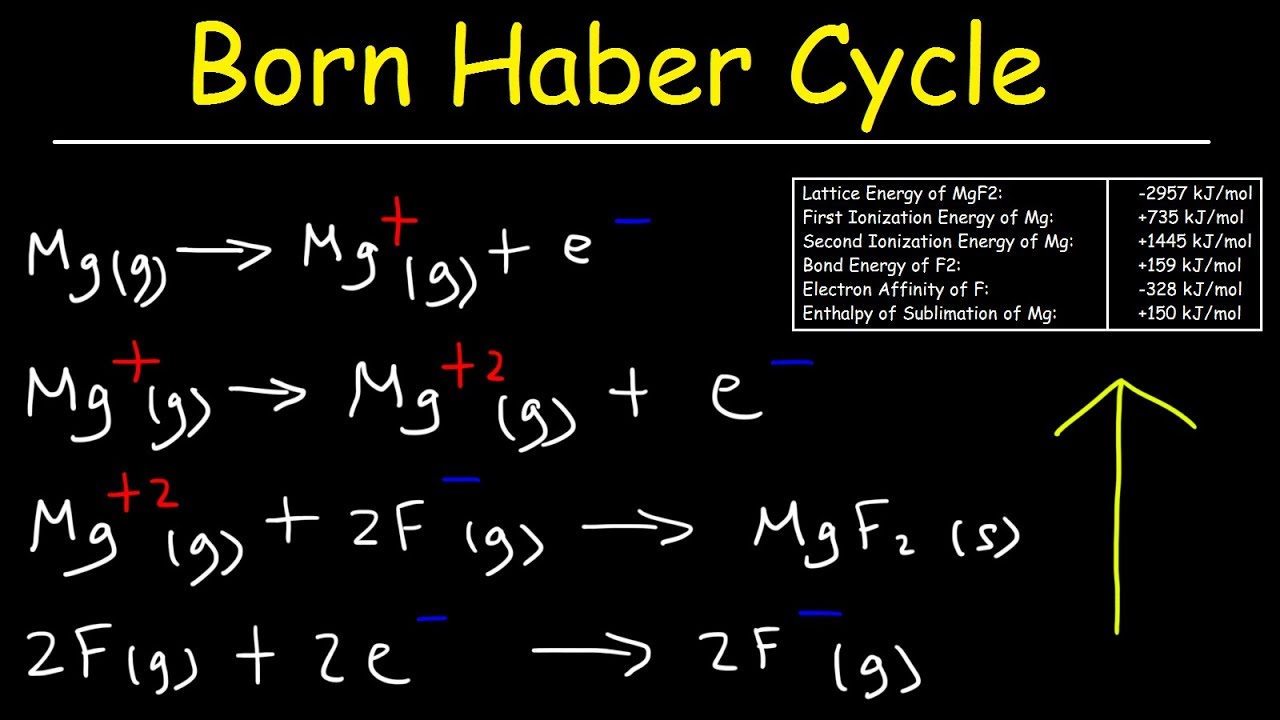
Born Haber Cycle, Basic Introduction, Lattice Energy, Hess Law & Enthalpy of Formation - Chemistry - YouTube

SOLVED: Use the data provided below to calculate the lattice energy of RbCL Is this value greater or less than the lattice energy of NaCl? Explain Electron affinity of Cl = -349

Calculate the lattice enthalpy of KCl from the following data by Born- Haber's Cycle. Enthalpy of sublimation of K = 89 kJ mol^(–1) Enthalpy of dissociation of Cl = 244 kJ mol^(–1)