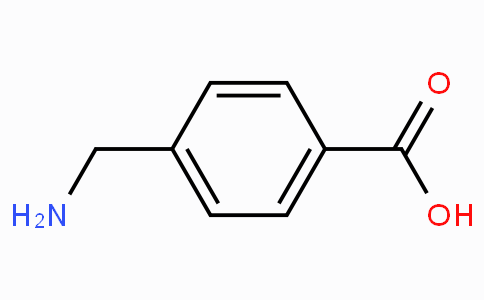
1) Rank trans-stilbene, benzoic acid, and fluorenone by polarity (least polar to most polar). Provide an explanation for your proposed ranking. (2) Hexane, ethyl acetate and acetone will be used as
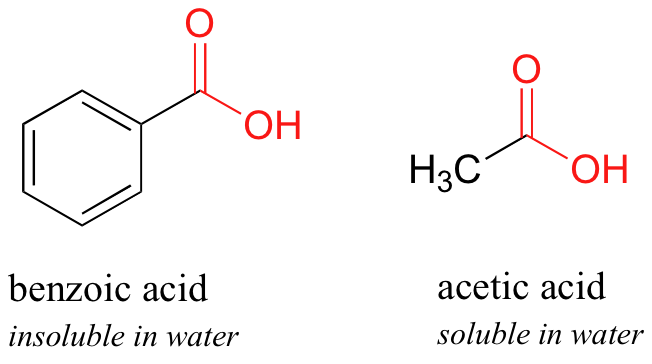
Between acetylsalicylic acid and benzoic acid, which is most polar if you look only at the structure (not at solubility in water g/l), and why? Why is that structure most polar? -
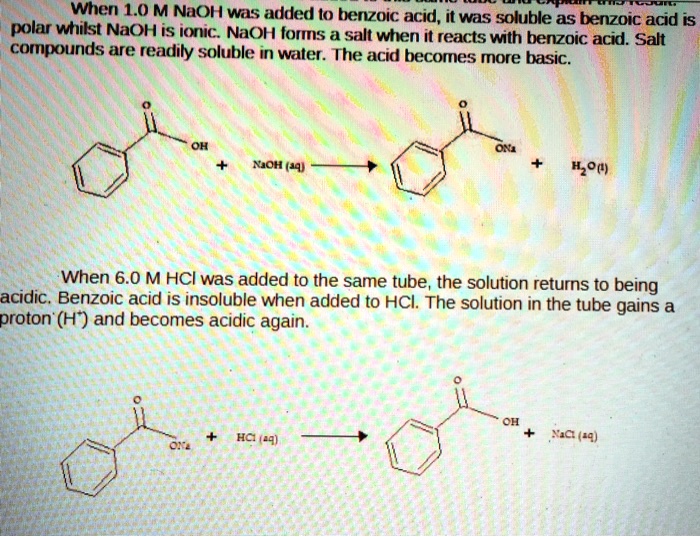
SOLVED: When 1.0 M NaOH was added (0 benzoic acid, it was soluble as benzoic acid is polar wtitst NaOH is ioric; NaOH forms salt when i reacts with berizoic aczo Sad

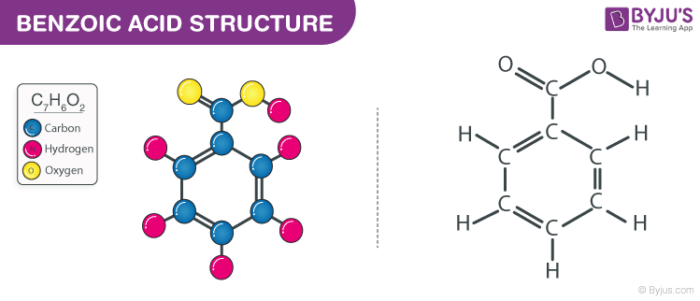
2. Draw the chemical structure of benzoic acid and use it to answer the following solubility-based questions: a) Circle and label the region of the molecule that exhibits non-polar behavior. b) Thes

Phototransformations of 2-(1,2,4-Triazol-3-yl)benzoic Acid in Low Temperature Matrices | The Journal of Physical Chemistry A